Preventing Pandemics: A Closer Look at Potentially Threatening Animal-Borne Diseases
To many, the COVID-19 pandemic, social distancing, mask-wearing, and vaccinations are a thing of the past; a historic event that will be retold countless times to eager-or exasperated- grandchildren in a half century. Despite the triumph of modern science and the development of a successful vaccine in an unprecedentedly short period, Pandemics remains a great threat in our modern global society. The very technologies that allow for rapid transportation and convenient services - hallmarks of our vision of advanced society- can also act as effective media to rapidly propagate novel diseases in a shockingly brief span of time. Making strides toward preventing and managing future Pandemics, a research team led by Xinyuan Cui investigated the source of some of the most likely perpetrators of a future outbreak.
Zoonotic Diseases
Any familiar with media coverage of the COVID-19 pandemic are likely aware of numerous explanations for the origin of the infamous SARS-COV-2. Explanations have ranged from radical conspiracy theories with no backing to highly plausible explanations substantiated by scientific evidence and peer-reviewed literature. While some have claimed that SARS-COV-2 was developed intentionally and inadvertently released from a biotechnology laboratory, there is scant evidence to support this conjecture. Rather, many scientists believe that SARS-COV-2 and some other recent pandemics may have originated from wild animals.
Horseshoe bats (above) have been identified as carriers of coronaviruses that have the potential to become infectious to humans. Some have postulated that SARS-COV-2 may have originated from a bat species. Image credit: Frank Greenaway.
While it may seem unusual that human diseases can originate in animals, zoonotic diseases (diseases spread from animals to humans) are not entirely uncommon. When humans first began raising livestock thousands of years ago, it is thought that they acquired numerous diseases from domesticated animals. Centuries of exposure to zoonotic diseases are thought to have conferred resistance to societies in much of the Old World that were reliant on livestock. During the Columbian Exchange, indigenous people of the Americas did not raise livestock, a factor that may have contributed to their lower tolerance for diseases imported by settlers from the Old World. As such, zoonotic diseases may have played a role in human society thousands of years before present.
While diseases from livestock continue to present healthcare challenges, modern scientific understanding and advanced scientific understanding have reduced this threat. Far more alarming are zoonotic diseases that arise from wild animals. Diseases borne by wild animals pose a far greater threat as many are poorly understood and the transition to a zoonotic disease can occur in parts of the world where proper sanitation protocols are not followed. Two recent outbreaks caused by the coronaviruses Severe Acute Respiratory Syndrome (SARS-CoV) and Middle East Respiratory Syndrome (MERS-CoV) are known to have originated from bat viruses. SARS-CoV-2 itself may have found its origin in bats as well, but the source of the deadly pathogen is still debated. Even so, considerable evidence demonstrates the threat zoonotic diseases from wild animals can pose to public health. Xinyuan Cui and colleagues captured examples of various mammal species from different locations in southern China to analyze any potential pathogens they carried. Samples of saliva, body fluids, and fecal matter were collected from bats, rodents, pikas, pangolins, insectivorous mammals, and zoo animals. RNA was extracted from the samples and analyzed to gain insight on the characteristics of any diseases present and the relationships among sequenced strands.
Pangolins have been identified as carriers of various diseases that may be transferable to humans. Image credit: iStock.
Although zoonotic diseases are very prevalent and may be behind major outbreaks, they may seem strange in a world where most only encounter diseases transmitted between people. How can a disease suddenly switch from only infecting a certain group of animals to infecting another - including people? To investigate the roots of zoonotic diseases, one must first gain an understanding of one of the key mechanisms behind evolution: mutations.
The Biology: A Look at Mutations
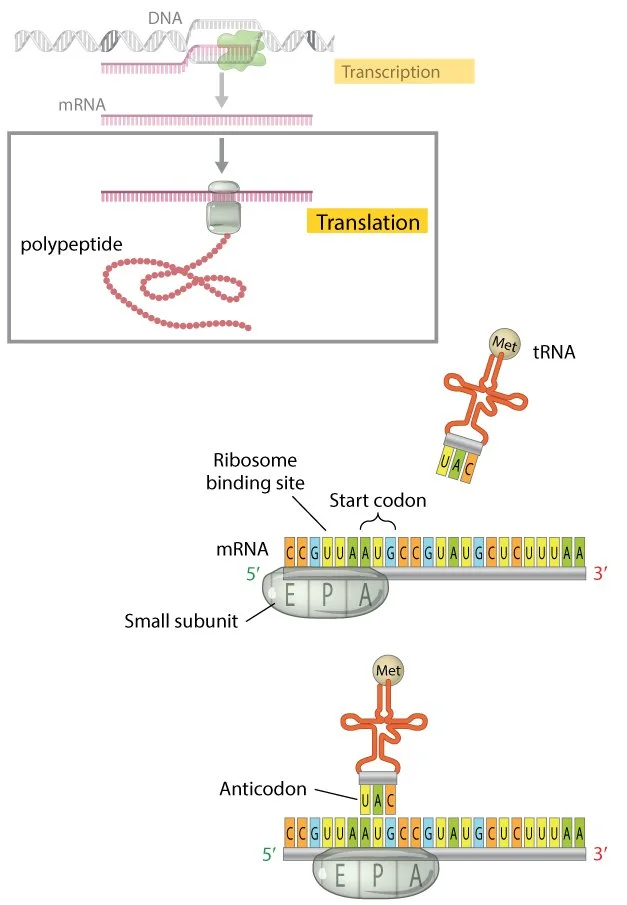
As any intro biology student would quickly recite, biological organisms operate following what is termed the central dogma: deoxyribonucleic acid (DNA) gives rise to ribonucleic acid (RNA) which in turn gives rise to proteins. All cellular processes rely on the central dogma, a process also known as transcription and translation. During transcription DNA, often known as the ‘instruction manual’ of the cell is copied through the synthesis of a nucleic acid known as mRNA. Each strand of mRNA, which corresponds to a specific segment of DNA known as a gene, is used to assemble a protein from amino acids in a process known as translation. The conversion of information stored in DNA to physical proteins is essential to all cellular processes, yet, as with all aspects of nature, the process is far from perfect.
Occasional errors do occur in transcription and translation that can result in a handful of malformed proteins, but the most significant errors take place at the source: DNA. As the starting point of protein synthesis, DNA contains all the information required to produce the myriad proteins needed to maintain cellular metabolisms. Any errors or changes in DNA affect any proteins produced, leading to sustained effects within the cell, and at times across the entire organism.
DNA replication is the complex process in which one strand of DNA gives rise to two identical strands - or does it? DNA replication typically does lead to two identical strands, but errors in DNA replication are one source of mutations. Image credit: Alicia Cotoia.
Changes in the DNA sequences, better known as mutations, are varied in nature and have a wide variety of causes. Some mutations are caused by endogenous (internal) factors. When a cell divides in the process of mitosis, copies of DNA are produced in the process of DNA replication to provide both daughter cells with identical sets of genetic information. Errors in DNA replication that escape corrective measures can be the basis of mutations. Natural and essential processes of cellular metabolisms can also give rise to byproducts that can cause mutations. Mitochondria, often termed the powerhouses of the cell, produce highly reactive compounds known as reactive oxygen species (ROS) as they produce ATP to power cellular processes. Due to their high reactivity, ROS can interfere with DNA and give rise to mutations. Mutations can also be caused by exogenous (external) factors. Exogenous causes of mutations include radiation and certain varieties of chemical compounds. Just as there are numerous factors that can cause mutations, mutations themselves are inherently diverse. To better understand and study mutations, they are often classified into several basic groups depending on the general change that occurs within DNA.
Point Mutations
The first broad category of mutations are point mutations, which, as the name implies, occur at a specific location in the DNA sequence. When a point mutation occurs, a single nucleotide in the DNA sequence is removed and replaced by a different nucleotide. As a result, the genetic sequence differs by one letter - or a variable number of letters if more than one point mutation were to occur. To give a basic example, we will consider a single gene that has one point mutation. As the cell prepares to synthesize the specific protein associated with that gene, the transcription process produces a strand of mRNA with one changed nucleotide. The altered mRNA is translated, and various effects can be observed on the resultant polypeptide depending on the circumstances. When translation takes place, the mRNA is ‘read’ in segments consisting of three nucleotides. Each of these segments, known as codons, correspond to or ‘code for’ a specific amino acid. As such, the point mutation can only impact one amino acid within the larger polypeptide.
Translation (as depicted above) relies on transfer RNA molecules that bring the appropriate amino acids based on 3-nucleotide codons. A point mutation often changes the amino acid that is brought and incorporated into the polypeptide. Image credit: Nature Education.
In some cases, the changed nucleotide in the mRNA sequence will not cause a change in the amino acid added to the polypeptide. Although the codon has changed, the inherent redundancy in amino acid coding can cause several codons to correspond to the same amino acid. Redundancy occurs since there are only 20 amino acids, but the 3-nucleotide codons and the presence of 4 distinct nucleotides in RNA can produce 64 unique combinations. When a point mutation leads to the expression of the same amino acid - and no change in the polypeptide and final protein - it is classified as a silent mutation since there is no impact on the organism’s phenotype. However, in many cases the nucleotide change produces a codon that corresponds to a different amino acid from that which would be present in mRNA transcribed from the original, unaltered DNA sequence. As a result, a new amino acid is incorporated into the polypeptide, a change known as a missense mutation.
Proteins are complex assemblages of polypeptides with various levels of structure. Mutations can alter the structure and function of proteins. Image credit: Scott Camazine.
Missense mutations can have a notable effect on the polypeptides produced through translation. When polypeptides assemble into complex proteins, the specific folding pattern of the protein is essential to the function it performs. Protein folding is ultimately reliant on the protein’s constituent amino acids, as their properties lead to forces of attraction and repulsion that determine the final shape. When a missense point mutation introduces a new amino acid, it can have different properties that cause it to experience different interactions with surrounding amino acids. Such interactions can have a considerable impact on the shape of the protein and can ultimately render it nonfunctional.
Frameshift Mutations
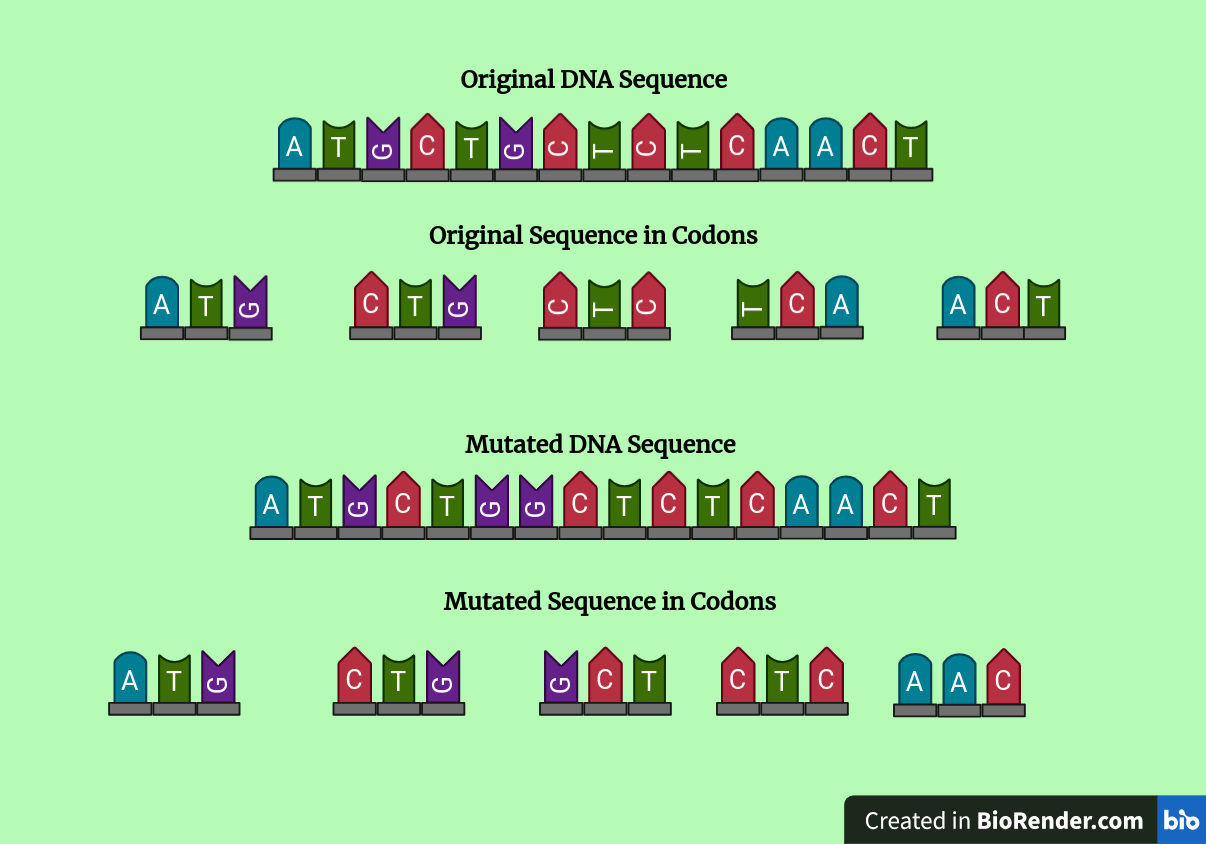
While missense point mutations can disrupt cellular processes through small alterations of protein structure, a second type of mutation has far more drastic effects. Frameshift mutations are mutations in which amino acids are either removed from (deletions) or added to (insertions) the genetic sequence. In both scenarios, a change in the total number of amino acids contained within the gene shifts the codon pairings and as such the ‘reading frame’ following the mutation is completely changed. Unlike point mutations, the effects of frameshift mutations are not relegated to one amino acid. Instead, they typically impact all amino acids following the mutation and often lead to a disastrous chain of unintended amino acids that produce a nonfunctional protein. To understand frameshift mutations, let us consider a gene that has experienced the insertion of one amino acid. Suppose that our gene has the nucleotide sequence shown in the diagram below.
A frameshift mutation visualized as a continuous DNA sequence and broken into codons. Image created using Biorender.
As can be seen in the mutated sequence, a guanine nucleotide was inserted into the original gene. Comparing the codons corresponding to each DNA sequence, the two codons that precede the insertion of the guanine nucleotide are identical. However, once the guanine is inserted, the codon incorporating the new nucleotide and all subsequent nucleotides have shifted over by one nucleotide. Each of the changed nucleotides likely corresponds to a new amino acid, and the resulting polypeptide and final protein will be misfolded. As such, the protein will be unable to perform its function and may cause a phenotypic change. Frameshift mutations can also lead to the formation of truncated or excessively long proteins if the frameshift creates or disrupts a stop codon. When mRNA strands are translated, several codon sequences do not code for an amino acid, instead signaling the termination of the polypeptide. These are known as stop codons. If a frameshift mutation forms a stop codon earlier in the sequence, the polypeptide will be shortened and nonfunctional. Similarly, if the mutation disrupts an existing stop codon it will cause the formation of an excessively long polypeptide that is likely to lose its intended shape and function.
Mutations are diverse and play a key role in evolution and natural variation in organisms. Point mutations and frameshift mutations occur at the nucleotide level, yet the impacts they have on resulting polypeptides and proteins can have notable impacts on the phenotype of the organism. In the case of viruses, such as those carried by the taxa examined by Cui’s team, random mutations in the genetic sequence can lead to the production of altered proteins. In certain cases, these altered proteins can allow viruses to interact with the cells of a new species, such as humans. The random nature of many mutations and the difficulty of predicting when zoonotic diseases may arise make the study of these illnesses an important focus of public health research.
The Findings
Cui and colleagues analyzed samples taken from each mammalian group and sequenced viral RNA. Metatranscriptomics, a field focused on detecting pathogens and other microbes from genetic material in environmental samples, was applied for isolation and sequencing of viral RNA. Genetic information was compiled into distinct libraries for each group of organism and further analyses were conducted using the software BLASTn and DIAMOND. Refined sequences were then compared using multiple sequence alignments and phylogenetic trees were generated to show the evolutionary relationships between the pathogens discovered.
Figure 5b of Cui et al. (2023) depicting a phylogenetic tree of the viral family Paramyxoviridae.
Phylogenetic trees were generated for several viral families identified in the study and for larger groups of viruses. Cui’s team successfully identified several undescribed viruses that were encountered during sample analysis and assessed relationships between viruses found in different temporal and geographic ranges within the same carrier species. Notably, the researchers found that certain diseases that affected pikas were transmitted to rodents and other contemporary mammals. Furthermore, they identified certain diseases that were transmitted from domesticated pigs to tigers which engaged in a predator-prey relationship. The team noted the importance of observed viral transmission between species and its relevance to the emergence of zoonotic viruses from diseases afflicting wild animals. Cui and colleagues emphasize the importance of studying viruses in wild animal populations to prevent the onset of future pandemics. To learn more, view the full publication in the journal Nature.